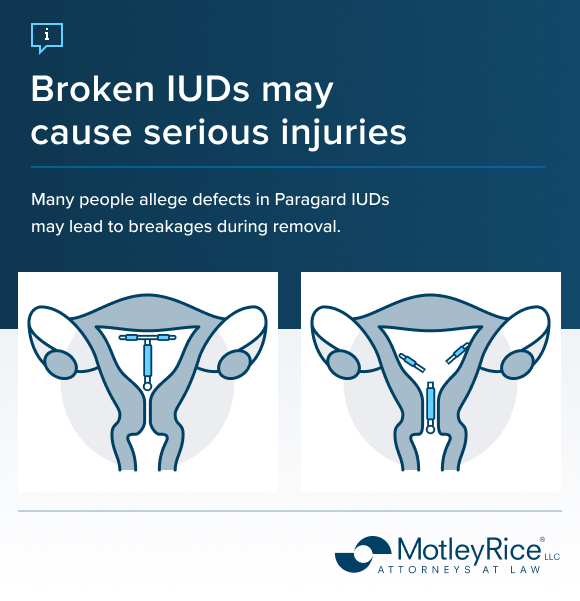
Thousands of lawsuits allege that the Paragard IUD can fracture when doctors attempt to remove it, and that the manufacturers failed to warn patients about this risk. Instead of sliding out intact, one or both of the device’s arms may snap off, leaving fragments lodged in the uterus or migrating to other parts of the body. These incidents have led to painful complications, repeat medical procedures and, in some cases, permanent injuries.
Patients have reported a range of complications linked to Paragard breakage, including:
- Infertility: Scarring and damage to reproductive organs may prevent future pregnancies.
- Infections: Embedded fragments can lead to pelvic inflammatory disease (PID) or chronic infections.
- Organ damage: Broken pieces may migrate into the abdomen or pelvis, injuring organs such as the bladder or intestines.
- Pregnancy complications: Breakage has been associated with miscarriage and ectopic pregnancy.
- Surgical intervention: Some patients required hysteroscopy, laparoscopy or even a hysterectomy to retrieve fragments.
- Uterine perforation: Device fragments can puncture the uterine wall.
People with Paragard IUDs and their medical providers have made more than 54,000 reports to the Food and Drug Administration (FDA) since Paragard was FDA-approved in 1984, according to publicly available data in the FDA Adverse Event Reporting System (FAERS) database.
Of the more than 54,000 reports, nearly 24,000 have involved serious health problems, as classified by the FDA. According to the FDA, by the second half of 2025, there had also been 22 related deaths.
*FAERS is intended to help identify safety concerns related to marketed products, but it does not prove any product or drug is linked or caused a particular side effect or injury. Reports by themselves are not an indicator of a medical product’s safety profile. In addition, FAERS may include duplicate reports or may significantly undercount injuries.