
The number of pending cases in the MDL continued to grow. There are now 1,521 plaintiffs in the semaglutide lawsuits.
This is an Active case
Semaglutide lawsuits are being filed by patients who are experiencing serious health effects that were not listed on the warning labels of Ozempic®, Wegovy® or Rybelsus®.
Connect with an attorney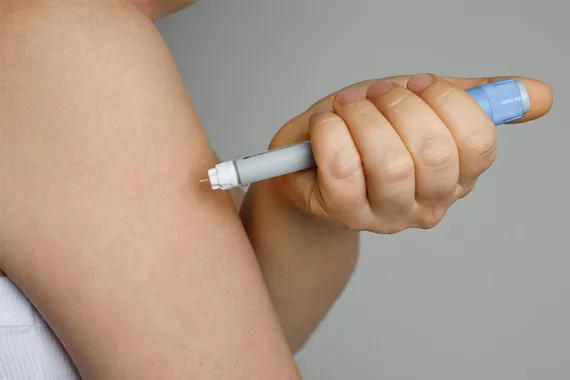
Semaglutide is the generic name for a drug used to treat diabetes and manage weight. It has gained popularity under various brand names. Ozempic® and Rybelsus® are approved to help manage type 2 diabetes, while Wegovy® is approved to help manage obesity. Patients using semaglutide have experienced unadvertised side effects affecting the eyes, digestive system and vascular system.
Semaglutide is a type of diabetes medicine that the U.S. Food and Drug Administration (FDA) has approved the drug for treating type 2 diabetes and obesity. As a glucagon-like peptide-1 receptor agonist (GLP-1 RA), semaglutide increases insulin production. This helps decrease the amount of sugar that enters the bloodstream.
The FDA currently has approved three forms of semaglutide:
Novo Nordisk manufactures all three forms of the drug. Ozempic and Wegovy are injections that patients take once a week. Rybelsus is a tablet that patients take daily. Doctors prescribe dosages and timeframes based on each patient’s condition and tolerance for the medication.
Semaglutide has become a popular weight loss medication. Wegovy is FDA-approved to help people struggling with weight-related conditions. Doctors may also prescribe Ozempic and Rybelsus for off-label use to help people lose weight.
The FDA recommends that patients supplement all semaglutide medications with exercise and diet changes to achieve the best results.
Semaglutide drugs Ozempic and Wegovy were on the FDA’s drug shortage list for several years. The diabetes medication shortage was likely linked to its popularity as a weight loss treatment. A compounded version of the medicine — made with semaglutide and other ingredients — was made available for some patients to combat the shortage. The compounded version of semaglutide may also be dangerous, but is not involved in the lawsuits against the manufacturers.
All medicines may cause side effects. But semaglutide drugs like Ozempic and Wegovy may carry additional risks beyond the ones listed on the drugs’ warning labels. Some reports allege people have experienced the following health problems after taking semaglutide:
People who experienced these side effects are filing semaglutide lawsuits. Lawsuits against drug manufacturers can help people receive money to pay for medical expenses, increase labeling requirements and even prevent other people from experiencing similar harms.
If you or a family member have experienced serious side effects from semaglutide, you might be eligible to seek compensation. For more information, contact our team by filling out our online form or call 1.800.768.4026.
While the compounded versions of Semaglutide drugs are legal, they do not have FDA approval like the non-compounded versions do. Keep in mind, these drugs are not eligible for GLP-1 lawsuits. The FDA has said that it “has received adverse event reports after patients used compounded semaglutide.”
One report in the Journal of the American Pharmaceutical Association found severe adverse effects for three people who took compounded semaglutide. The patients who took the compounded semaglutide experienced a number of side effects, including:
For all three people, their symptoms persisted for days after taking the compounded semaglutide.
The FDA has expressed concerns that compounding pharmacies are fulfilling orders using semaglutide salts—variations on the semaglutide formulation approved by the FDA for Ozempic and Wegovy. In a letter to the National Association of Boards of Pharmacy, the FDA warned that the agency has not evaluated semaglutide acetate or semaglutide sodium—two types of semaglutide salts—for safety or efficacy. It also noted that the use of semaglutide salts may be a violation of the Federal Food, Drug, and Cosmetic Act (FD&C Act).
If you’ve received compounded semaglutide and are concerned you may be experiencing an adverse reaction, please seek immediate medical attention. Do not stop taking a prescribed medication without speaking to a medical professional first.
If you or a loved one have experienced side effects that you believe are related to semaglutide, you may be able to seek justice through a semaglutide lawsuit. The following semaglutide lawsuits are currently pending:
People are suing Novo Nordisk for side effects they allegedly experienced after taking its semaglutide products, Ozempic, Rybelsus and Wegovy. These side effects may include those not included on either drug’s warning label.
Our law firm has filed a lawsuit for a Pennsylvania woman who was hospitalized for gastroparesis after taking Wegovy. Learn more about her case here.
Many of the lawsuits are part of a multidistrict litigation (MDL) involving other similar medications called GLP-1 RAs. Defendants in the MDL include Novo Nordisk, which manufactures Ozempic, Rybelsus and Wegovy. Another defendant is Eli Lilly, which makes Trulicity®, Zepbound® and Mounjaro®. All MDL cases involve patients who took one or more GLP-1 RA medications and experienced severe unlisted side effects.
If you or a family member have experienced a similar reaction after taking a semaglutide product, reach out to one of our attorneys to find out about the legal options you may have.
As of February 2025, there is no class action lawsuit against Novo Nordisk, the sole manufacturer of semaglutide in the U.S.
Instead, people who believe they’ve been harmed by unlisted side effects of semaglutide may consider filing an individual lawsuit against Novo Nordisk. The drug manufacturer is currently facing multidistrict litigation (MDL) filed in federal district court for the Eastern District of Pennsylvania.
These lawsuits allege Novo Nordisk failed to warn patients about the risks of taking Ozempic and other semaglutide drugs.
03.03.25
The number of pending cases in the MDL continued to grow. There are now 1,521 plaintiffs in the semaglutide lawsuits.
02.21.25
Semaglutide injections were removed from the FDA Drug Shortages List. The drug was on the list starting in 2022.
The end of the drug shortage may impact compounding pharmacies. Now that semaglutide is no longer in a shortage, the compounding authorization is essentially revoked. People receiving compounded semaglutide drugs may have to find a different route to get their medication.
02.20.25
An analysis of 37.1 million adults with type 2 diabetes across 14 databases found “further evidence of an association between semaglutide and NAION.”
01.30.25
A small study focused on nine participants taking semaglutide and tirzepatide, a similar drug.
•Seven developed NAION
•One developed a stroke in the retina (paracentral acute middle maculopathy)
•One developed swelling in both optic nerves (bilateral papillitis)
12.01.24
Deep vein thrombosis and blood clot cases will be excluded injuries in the GLP-1 RA MDL. However, people who suffered these injuries can still pursue these cases as separate lawsuits. For more information, contact Motley Rice attorneys Sara Couch or Jonathan Orent.
11.01.24
Patients who took Ozempic filed a master complaint in the growing multidistrict litigation centralized in the Eastern District of Pennsylvania. They allege Novo Nordisk and Eli Lilly & Co. failed to warn them about the risks associated with Ozempic and other comparable GLP-1 drugs.
11.01.24
The FDA included an additional warning on the Ozempic label regarding an increased risk of pulmonary aspiration. The warning indicates some Ozempic users may experience the issue during general anesthesia or deep sedation, even after following preoperative fasting directives.
10.02.24
Semaglutide drugs remained on the FDA Drug Shortage Database, although drugmakers were increasing production to meet the demand. The shortage made it difficult for patients using semaglutide drugs for diabetes to obtain their medications. As of October 2, 2024, tirzepatide drugs were removed from the FDA Drug Shortage Database.
09.05.24
The CEO of Novo Nordisk appeared before a U.S. Senate committee to address Senate concerns regarding overly high pricing for Ozempic and Wegovy. According to Senator Bernie Sanders, the manufacturer charged Americans higher prices than customers in other countries.
09.04.24
A new plaintiff, Juanita Gantt*, joined the GLP-1 RA medications MDL, alleging life-threatening health issues related to Wegovy and Ozempic.
While taking the GLP-1RA medications, Gantt was hospitalized and required emergency surgery to remove part of her large intestine. She suffered cardiac arrest while recovering from surgery and required an ileostomy bag to collect waste from her body.
She Gantt’s lawsuit claimed Novo Nordisk failed to warn patients about the risk of major gastrointestinal side effects connected to the use of GLP-1RA drugs.
*Motley Rice does not represent Ms. Gantt.
08.01.24
Judge Karen Spencer Marston issued Case Management Order No. 17 to establish guidelines regarding how plaintiffs will share expenses incurred by their attorneys while performing MDL-related duties.
07.01.24
A new study linked semaglutide drugs like Ozempic to non-arteritic anterior ischemic optic neuropathy (NAION), a side effect that causes permanent vision loss and blindness. NAION involves blood loss to the optic nerve that can lead to visual impairments and blindness. The researchers’ findings suggested an association between semaglutide and NAION.
06.01.24
Judge Karen Marston was appointed to head the MDL following Judge Pratter’s death in May. Facing a complex litigation with competing interests from all sides, one of Judge Marston’s first acts was to request a status conference with leadership members.
06.01.24
Science Day, set for September 4, 2024, helps establish facts and relationships between the drugs, current research and the plaintiff’s claims.
05.01.24
Judge Gene E.K. Pratter, who oversaw the GLP-1 RA litigation, passed away at 78. The Philadelphia Inquirer remembered Judge Pratter as a “longtime lawyer, professor, mentor and volunteer.” The Judicial Panel on multidistrict Litigation (JPML) will appoint another judge to take Judge Pratter’s place in the GLP-1RA MDL.
05.01.24
Motley Rice attorney Jonathan Orent was appointed Co-Lead Counsel and Motley Rice attorney Sara Couch was appointed Chair of Marketing Discovery for the Ozempic multidistrict litigation’s Plaintiffs’ Executive Committee.
04.01.24
Judge Pratter appointed a Motley Rice lawyer and attorneys from other firms to a “plaintiffs’ committee” to guide and lead the Ozempic MDL.
03.01.24
The first status conference in the GLP-1 RA MDL was held on March 14 to handle procedural details, leadership structure and the establishment of facts for the litigation.
02.01.24
A GLP-1 RA MDL was established in the Eastern District of Pennsylvania. U.S. District. Judge Gene E.K. Pratter was named the presiding judge. Louisiana lawsuits were transferred to the Pennsylvania MDL.
01.01.24
The FDA announced it was investigating claims of suicidal thoughts, hair loss and intraoperative pulmonary aspiration linked to the use of GLP-1 RAs.
12.31.23
6,487 total cases, including 89 deaths.
12.01.23
Plaintiff lawyers petitioned the court to consolidate nine GLP-1 lawsuits into multidistrict litigation in Louisiana.
12.01.23
Judge James Cain, Jr. denied Novo Nordisk’s motion to dismiss, granted the dismissal of express warranty claims and denied the failure to warn claims.
11.01.23
Novo Nordisk filed a motion to dismiss the Bjorklund lawsuit.
09.01.23
Ozempic’s label was updated to include a warning for ileus in the postmarketing experiences section.
08.01.23
Summons were issued to Novo Nordisk and Eli Lilly in the Bjorklund case.
08.01.23
Forty-four-year-old Jaclyn Bjorklund filed one of the first Ozempic lawsuits against Novo Nordisk et al. The suit alleged that the drugmaker’s medications Ozempic and Mounjaro caused her to develop gastroparesis.
Our medical attorneys have represented thousands of patients seriously hurt by dangerous prescription and over-the-counter drugs. We understand that as a patient or family member, what you know about your or a loved one’s medications may be limited.
If you believe a medicine made you sick or hurt you, our attorneys have the resources needed to thoroughly investigate and:
Learn more about our medical drug litigation experience here.
Do not stop taking a prescribed medication without first consulting with your doctor. Discontinuing a prescribed medication without your doctor’s advice can result in injury or death. Ozempic, Wegovy, Rybelsus, Trulicity, Zepbound and Mounjaro drugs remain approved by the U.S. Food and Drug Administration.
Important semaglutide lawsuit updates
Key takeaways about semaglutide lawsuits
What is semaglutide?
Potential dangers of semaglutide
Current semaglutide lawsuits
Semaglutide lawsuit updates timeline
Our medical drug litigation experience
Call us or fill out our online form with the details of your potential case.
Our team reviews your information to assess your potential case.
Talk with us about next steps.