
The talcum powder MDL grew to 67,622 cases.
This is an Active case
Talcum powder lawsuits allege that the use of talcum powder products increases the risk of developing ovarian cancer and other diseases.
Connect with an attorney
Research has found that using products containing talcum powder in the genital area may increase the risk of developing ovarian cancer and other diseases. One example of a talcum powder product is Johnson & Johnson’s baby powder. If you or a loved one have suffered a health condition that you believe is linked to using products with talc, you may be eligible to file a talcum powder lawsuit.
People are filing talcum powder lawsuits against Johnson & Johnson (J&J) and associated companies for selling talcum powder products that allegedly lead to the development of ovarian cancer. In these cases, women and their families allege that regular and prolonged exposure to products like Johnson & Johnson Baby Powder caused their cancer.
Other plaintiffs have alleged that they’ve developed cancers like fallopian tube cancer and mesothelioma. Mesothelioma may develop after exposure to asbestos-contaminated talc.
Plaintiffs in these talcum powder lawsuits seek compensatory and punitive damages for the injuries that have disrupted their lives.
As of February 2, 2026, 67,622 plaintiffs have filed lawsuits as part of a multidistrict litigation (MDL) action in federal court. These cases are coordinated for pretrial discovery in In re Johnson & Johnson Talcum Powder Products Marketing, Sales Practices and Products Liability Litigation, MDL No. 2738, overseen by Judge Michael A. Shipp.
Talc is a naturally occurring mineral mined from the earth. It is refined and milled to create talc products, including talcum powder, cosmetics, baby powder or body powder. People may use these products in their daily hygiene routine.
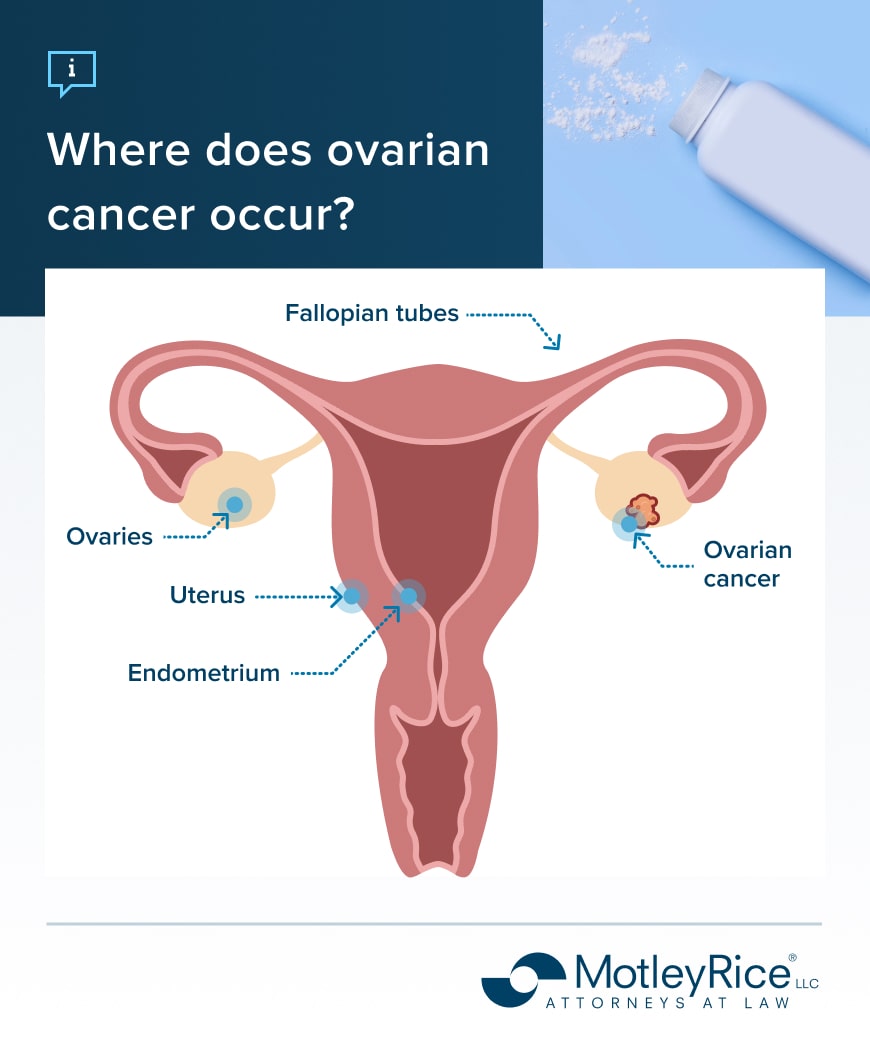
Using talcum powder on the genitals and perineal area has been associated with an increased risk of ovarian cancer. Scientists think talcum powder applied to the genital area may travel to the ovaries, causing inflammation. This may happen if the product is directly applied to the area or a sanitary napkin.
2.03.26
The talcum powder MDL grew to 67,622 cases.
1.05.26
The number of cases in the talcum powder MDL dropped to 67,580.
12.03.25
Throughout November, more plaintiffs joined the talcum powder MDL. There are now 67,670 pending actions.
11.03.25
The Judicial Panel for Multidistrict Litigation (JPML) issued an update on the number of plaintiffs in the talcum powder MDL. There were 67,229 pending actions heading into November.
10.01.25
The total number of cases in the J&J talcum powder MDL increased to 67,204.
09.02.25
As of September 2, 2025 the total number of cases in the J&J talcum powder MDL was 66,910.
08.01.25
There were 66,509 pending actions in the Johnson & Johnson Talcum Powder MDL, an increase of more than 2,800 lawsuits.
07.01.25
The total number of cases in the federal lawsuit docket was 63,693 as of July 1, 2025.
03.31.25
The third bankruptcy attempt by a Johnson & Johnson subsidiary was dismissed by a Texas judge. J&J has sought to use Chapter 11 bankruptcy to settle thousands of claims of plaintiffs who allege talc in J&J products caused their cancer.
03.18.25
Johnson & Johnson and its subsidiaries argued that it didn’t act with bad intentions and shouldn’t be responsible for paying a Connecticut man $30 million in extra damages for his lung cancer claims.
02.18.25
Trial began in Johnson & Johnson subsidiary, Red River Talc LLC’s attempt to resolve talc litigation through Chapter 11 bankruptcy. This $10 billion bankruptcy case went before a Texas judge.
02.04.25
The total number of cases in the docket was 58,206 as of February 3, 2025.
12.26.24
In response to the growing concern that talcum powder contains asbestos, the FDA announced new testing for cosmetic products made with talc. This new testing detects asbestos contamination in cosmetic products.
11.08.24
A group of insurance carriers that provided insurance coverage to Johnson & Johnson and its subsidiaries urged a Texas bankruptcy judge to reject the plan disclosure statement of J&J subsidiary, Red River Talc. According to the insurers, the disclosure statement — filed by Red River Talc— should be deemed “unconfirmable.” The insurance carriers also claimed that Johnson & Johnson has “made it clear that it intends to saddle its insurers with responsibility to pay.”
11.01.24
Plaintiffs continued to file J&J talcum powder lawsuits, bringing the total number of cases in the docket to 58,198 at the start of November.
10.01.24
Johnson & Johnson continued to use subsidiaries as a shield against talcum powder lawsuits and liabilities. Under its subsidiary Red River Talc LLC, Johnson & Johnson attempted to use Chapter 11 bankruptcy proceedings to resolve talc litigation. This was the company’s third attempt to use Chapter 11 bankruptcy filings in this way. The U.S. Trustee (part of the Department of Justice) called the tactics of Johnson & Johnson “a textbook example of bad faith.”
10.01.24
More plaintiffs affected by J&J’s talcum powder products filed cases, bringing the total number of actions in the MDL to 58,052 at the start of October.
07.01.24
Johnson & Johnson agrees to pay into Imerys’s bankruptcy fund. J&J agreed to pay $505 million into the talc supplier’s planned Chapter 11 bankruptcy trust. The proposed payment would still be made even if J&J attempts another bankruptcy petition of its own. A Delaware court will review the offer.
05.01.24
Johnson & Johnson announced its intent to solicit votes in support of a third bankruptcy filing of a subsidiary company that holds liability for talc-based claims. The solicitation seeks votes in support of a bankruptcy plan that would direct $6.48 billion to resolve all current and future lawsuits that claim the company’s talc-based products have caused injury.
01.01.23
The company’s plan to resolve talcum powder litigation by offloading the liability to a subsidiary was rejected. The Third Circuit Court of Appeals held that Johnson & Johnson subsidiary, LLT Management LLC, didn’t have a legitimate need to declare bankruptcy. This decision allowed cases against the company to resume.
08.11.22
Johnson & Johnson announced in 2022 that it would stop selling talc-based baby powder products worldwide in 2023. The company also announced that it would replace those products with cornstarch-based products, following the lead of other global companies.
09.19.22
The company’s plan to handle talcum powder cases by offloading them to a subsidiary was rejected. The Third Circuit Court of Appeals in Philadelphia found that Johnson & Johnson and its subsidiary didn’t have a legitimate need to declare bankruptcy. This decision allowed cases against the company to resume.
10.12.21
Johnson & Johnson created a subsidiary to handle talcum powder claims and liabilities. The subsidiary filed for bankruptcy two days after being created, devising a plan to create a trust fund to handle future claims. This plan is known as the “Texas Two Step” and is an effort by companies to sidestep their liabilities.
04.27.20
A U.S. District Court Judge in New Jersey ruled that thousands of women alleging Johnson & Johnson baby powder and other talc-based products caused their ovarian cancer could move forward with their claims. Johnson & Johnson had sought to bar expert testimony from trial. However, after hearing both plaintiffs’ and defendants’ arguments, U.S. District Court Judge Freda Wolfson ruled that experts would be allowed to testify in trial about studies showing a link between talcum powder exposure to genitals and ovarian cancer.
01.01.20
Johnson & Johnson announced in 2020 that it would end production of its talc-based baby powder products in the U.S. and Canada.
10.24.19
In October 2019, multiple major retailers removed all 22-ounce bottles of Johnson & Johnson Baby Powder from store shelves.
12.01.18
Previously confidential company documents indicate that Johnson & Johnson allegedly knew about the presence of asbestos in its talcum powder. A Reuters investigation showed that the company did not make these findings public for decades.
10.04.16
On October 4, 2016, cases against Johnson & Johnson were consolidated into multidistrict litigation in the U.S. District Court for the District of New Jersey. This MDL is In re Johnson & Johnson Talcum Powder Products Marketing, Sales Practices and Products Liability Litigation, MDL No. 2738.
02.22.16
Jacqueline Fox was diagnosed with ovarian cancer in 2013 and passed away in 2015 from the disease. She claimed her use of Johnson & Johnson’s baby powder and Shower to Shower powder led to her cancer diagnosis. The jury found Johnson & Johnson liable and awarded $10 million in actual damages and $62 million in punitive damages to the woman’s family.
12.04.09
The first talc lawsuit filed against Johnson & Johnson to reach a jury was filed by a South Dakota woman in 2009. Her case alleged the company’s baby powder products contributed to her ovarian cancer diagnosis. In 2013, a jury verdict found Johnson & Johnson negligent for its failure to warn women of an increased cancer risk from using its products.
You may be eligible to bring a personal injury lawsuit against manufacturers of talcum powder product if:
The most common defendants in talcum powder product cases are Johnson & Johnson and its various subsidiaries. They represent manufacturers and distributors of several talc-containing products.
Previous defendants include Imerys Talc America, Inc. and Personal Care Products Council (PCPC). Imerys is a talc mining and distribution company that supplied Johnson & Johnson’s talc. PCPC is an American trade organization for the cosmetics and personal care products industries. It influences governmental agencies on issues concerning the marketing and sales practices of these products. The council’s actions have had an impact on the talc product landscape in the U.S.
More than 63,000 people have filed lawsuits alleging harm caused by the genital use of talcum powder products. Because of the large volume of talc cases, federal district courts have coordinated many lawsuits into a single multidistrict litigation (MDL) docket. Coordination can ease the burden on district courts while still allowing injured parties to individually pursue justice for the harms they experience.
Mass tort refers to civil litigation where many people file individual lawsuits against common defendants for similar harms. Class actions and mass torts are two different types of litigation.
For talcum powder lawsuits, you can file an individual lawsuit that may become part of the MDL. Speaking with talcum powder lawsuit lawyers can help you understand your legal options.
Damages may help compensate individuals for their injuries. They may be informally called “payouts” or more accurately called "settlements" or "judegements" depending on how an agreement was reached. In talcum powder or baby powder lawsuits, “injury” encompasses ovarian cancers.
In a talc-related lawsuit, you may be eligible to sue for damages for:
In addition to the above damages, an individual may also be awarded “punitive damages.” This occurs when a court punishes a defendant for especially flagrant abuse of the law.
Plaintiffs who have filed talcum powder lawsuits against Johnson & Johnson have won both compensatory and punitive damages. For example, in 2018, a Missouri jury awarded 22 women and their families nearly $5 billion in compensatory and punitive damages from Johnson & Johnson. On appeal, a different court reduced the compensation. However, it still held that J&J was liable for the harm.
Any compensation you may receive depends on the unique facts of your case. It may also depend on whether you are able to settle your claims and what a judge or jury awards you if your case is tried. A legal professional with experience handling toxic exposure and medical claims can discuss what damages may be available to you.
A few talcum powder cancer settlements and jury awards include:
The plaintiffs in the above talc lawsuits were not represented by Motley Rice. The outcomes of these cases are not guarantees of any future settlement or jury award. The damages available may be different depending on the facts of your case. A talcum powder attorney can help you assess what compensation may be available to you.
If you used talcum powder and were diagnosed with ovarian cancer, you may have a case and may be eligible for compensation. For your case to proceed, you will need to show proof that:
Your lawyer can help you find, gather and organize all of this information. They will use it to build your potential case and file a lawsuit for you if you are eligible.
If you believe that you have a claim, consider contacting us as soon as possible. Depending on what state you live in, a statute of limitations (SOL) may limit the time you have to file a claim. If you do not file your claim within the SOL, you may lose the right to file in the future.
Motley Rice has experience litigating talcum powder lawsuits. Our team has been lead counsel in two separate trials litigating the harms caused by talcum powder.
Motley Rice attorneys Daniel Lapinski and Carmen Scott are members of the Plaintiffs’ Steering Committee (PSC) for the current federal multidistrict litigation (MDL). This committee is for cases alleging Johnson’s Baby Powder and other talc-based products caused ovarian cancer.
Dan also serves as co-chair of the Law & Briefing Committee, and Carmen is co-chair of the Bellwether Selection Committee. As of February 2, 2026, 67,622 cases were pending in federal court for the MDL, In Re: Johnson & Johnson Talcum Powder Products Marketing, Sales Practices and Products Liability Litigation.
The International Agency for Research on Cancer (IARC) is an intergovernmental agency that researches the causes of cancer. It classifies using talc-based body powder around the genitals as possibly carcinogenic to humans. This classification was influenced by years of research on the increased risk of ovarian cancer for people who apply talcum powder to sanitary napkins or directly to their genitals.
Studies point to the potential increased cancer risk from using talcum powder products. For example:
Dozens of other studies, analyses and meta-analyses have found similar results. Talcum powder use is linked to ovarian cancer, and the more a person uses it, the greater their risk.

Studies on how exactly talcum powder products lead to cancer are still ongoing. Some studies have found that talc affects cells during their re-oxygenation state. Other studies have suggested that asbestos contamination in talc products could lead to cancer in some people.
One 2020 study linked 75 cases of mesothelioma cancer to exposure to talcum powder contaminated with asbestos, a known carcinogen. Natural asbestos deposits may occur near talc, the mineral used for talcum powder. The physical proximity between asbestos and talc makes contamination possible.
Other studies have shown that talcum powder products are dangerous whether or not they are contaminated with asbestos. In the early 1990s, the United States National Toxicology Program published a study on the toxicity of non-asbestos form talc. The study found talc is carcinogenic, even without asbestos fibers present.
2024
The International Agency for Research on Cancer (IARC) reviewed evidence on the potential link between talc and cancer. They declared talc as “probably carcinogenic to humans.”
2016
The FDA implemented a full ban on powdered surgical gloves. The agency did so because of the clear evidence that powdered gloves represented a risk to patients.
2013
Cancer Prevention Research published a study that suggested an increased risk of ovarian cancer for women using talcum powder near their groin. The study found the risk to be between 20% to 30% higher for these women.
2010
The World Health Organization (WHO) International Association for the Research of Cancer (IARC) published a paper that labeled perineal use of talcum powder a human carcinogen. The IARC decided to label talc used around female genitals as a Group 2B human carcinogen after discovering a 30% to 60% increased risk of ovarian cancer among women who regularly used talcum powder.
2006
Imerys Talc began including potential cancer risk warnings on the Material Safety Data Sheets (MSDS) it included when selling talc to Johnson & Johnson.
1996
The FDA recommended that the condom industry stop the practice of dusting condoms with talc over concerns about the health impact of talc. The condom industry agreed and stopped producing condoms with talc.
1994
The Cancer Prevention Coalition sent a letter to Johnson & Johnson over concerns of talcum powder contributing to ovarian cancer risk. The letter suggested studies dating back to the 1960s provided evidence of the association. They requested that Johnson & Johnson either add a warning label to its talc products or replace them with cornstarch-based powders. The Cancer Prevention Coalition also petitioned the FDA, requesting a cancer warning label for cosmetic products that contained talc.
Early 1990s
The United States National Toxicology Program published research that found evidence of carcinogenic activity from talc. This study on the toxicity of talc suggested that talc was carcinogenic with or without the presence of asbestos-like fibers. The FDA asked manufacturers to remove talc from surgical gloves over growing evidence of adhesions — scar tissue that may form as a reaction to a foreign body — in surgical patients.
1986
Johnson & Johnson acknowledged concerns about some of its cosmetic products, noting the studies had found a link between female genital use of talc powder and ovarian cancer.
Early 1980s
The first epidemiological studies — studies that look at how conditions impact populations — found increased risks for ovarian cancer in those reporting female genital talc use. Shortly after the publication of the first study, Dr. Bruce Semple of Johnson & Johnson advised that the company should add a warning label to its talc products. More than 20 epidemiological studies published since the early 1980s further corroborate the link between talcum powder use and ovarian cancer.
1976
The Cosmetic, Toiletry and Fragrances Association (CTFA) announced new guidelines regarding talc use in United States cosmetic products. Any talc used in these products must be free from asbestos to protect consumers from cancer. However, these guidelines were voluntary.
1976
The Cosmetic Ingredient Review (CIR) was formed. This body was called an independent regulatory body for the cosmetic industry. However, it was funded entirely by the Personal Care Products Council (PCPC), a trade organization that has been heavily influenced by Johnson & Johnson and Imerys Talc.
Early 1970s
The first studies emerged suggesting a link between talc and ovarian cancer.
Watch Motley Rice attorney Carmen Scott discuss Johnson & Johnson discontinuing production of talc-based baby powder products in the U.S. and Canada:
The cases against Johnson & Johnson are some of the most highly publicized talc lawsuits in history and have been building for more than a decade.
Talcum powder has not been banned in the U.S., though Johnson & Johnson stopped producing talc-based baby powder globally as of 2023. It stopped selling talc-based baby powder in the U.S. and Canada in 2020 amid public concern over its reported link to ovarian cancer.
Talcum powder lawsuits under the multidistrict litigation (MDL) are still ongoing. This means that eligible people may be able to join others who have filed suits for injuries they’ve suffered from talcum powder use.
After having a third bankruptcy attempt dismissed, Johnson & Johnson says it’s now focused on litigating the talcum powder cases in the MDL.
The actual payout for a talcum powder lawsuit can vary substantially, based on the unique facts of the case. A few talcum powder settlements and jury awards have included amounts in the millions and billions of dollars. However, the outcomes of these cases aren’t guarantees of any future settlement or jury award.
A talcum powder attorney can help you assess what compensation may be available to you and help you comply with any talcum powder lawsuit filing deadlines.
No, Johnson & Johnson baby powder no longer contains talc as an ingredient. In 2023, the company stopped selling talc-based baby powder and replaced these products with cornstarch-based products.
Motley Rice attorneys lead litigation brought by thousands of women harmed by toxic exposure and defective products. Our law firm has extensive experience litigating product liability lawsuits for individuals who:
We also represent mothers suing over birth defects caused by prescription drugs and alleging necrotizing enterocolitis (NEC) linked to baby formula.
Read more on our women’s health litigation experience.
Do not stop taking prescribed medication without first consulting with your doctor. Discontinuing a prescribed medication without your doctor’s advice can result in injury or death. Paragard remains approved by the U.S. Food and Drug Administration.
Important talcum powder lawsuit updates
Key takeaways
What are talcum powder lawsuits?
News on Johnson & Johnson talcum powder lawsuits
Who qualifies for the talcum powder lawsuit?
Talcum powder and ovarian cancer links
Talcum powder and asbestos
Talcum powder and cancer research timeline
Talcum powder lawsuit lawyer discusses discontinuation of baby powder
Frequently asked questions about talcum powder lawsuits
Our experience representing women hurt by toxic exposure
Call us or fill out our online form with the details of your potential case.
Our team reviews your information to assess your potential case.
Talk with us about next steps.