
Case Overview
Ozempic® is a GLP-1 agonist drug intended to treat diabetes. It is also used off-label for weight loss. However, many expected and unexpected side effects are associated with using Ozempic. These risks can vary in severity, from severe vision loss and blindness to intestinal blockages and many other serious health conditions.
Key takeaways about Ozempic side effects
- Patients should be aware that Ozempic has both listed and unlisted potential side effects that range in severity.
- Listed side effects include, but are not limited to, hypoglycemia, pancreatitis and thyroid tumors.
- Unlisted potential side effects include, but are not limited to, vision loss, stomach paralysis and intestinal blockages.
What are the listed side effects of Ozempic?
Some of the most notable listed warnings and negative effects of Ozempic include organ injuries, thyroid cancer and tumors. Ozempic mimics hormones that reduce appetite, slow the movement of food and moderate insulin release. These effects can all change how the body functions.
Prescription medications include a label with applicable warnings about the medication. The warning label lists many restrictions, precautions and side effects of Ozempic.
Acute kidney injury
This condition may also be called acute renal failure. It can occur when the buildup of waste makes it difficult for your kidneys to maintain balance. Symptoms for acute kidney injury may include reduced urine output, swelling, seizures and coma.
Diabetic retinopathy
Diabetic retinopathy is an eye condition caused by high sugar levels in the blood. This condition can cause blindness and vision loss in people with diabetes. Diabetic retinopathy can lead to other serious conditions affecting the eyes. Symptoms for this condition may include blurry vision, vision loss and seeing dark areas, spots or floaters.
Some other vision changes have also been reported in people taking Ozempic that were not described on the warning label.
Gallbladder disease
Gallbladder disease describes any blockage or infection in the bile duct that backs up into your gallbladder. It can affect other digestive system organs as well. There are several types of gallbladder disease. Common symptoms of this disease include yellowing of the skin (jaundice), abdominal pain, fever, nausea and vomiting. Gallbladder disease was added to the Ozempic warning label in March 2022.
Hepatobiliary (cholecystitis)
This disease affects the digestive system (bile duct, liver, pancreas, gallbladder) and impacts how the body takes in nutrients and removes waste. Common symptoms of this disease might include abdominal pain, nausea, vomiting, loss of appetite and fatigue. Hepatobiliary was added to the Ozempic warning label. This occurred after the drug received FDA approval and after adverse health reactions were reported to the FDA (postmarketing experiences).
Hypersensitivity reactions
A hypersensitivity reaction is when the body has a certain response (usually extreme) to an antigen or allergen. Some hypersensitivity reactions symptoms are hives, stomach cramps, cardiac complications, rashes and many more. Hypersensitivity reactions were added to the Ozempic label after the FDA had approved the drug and had received negative postmarketing experience reports.
Hypoglycemia
Hypoglycemia means your blood sugar levels are lower than the standard range. Symptoms might include irritability, shakiness, illness, nausea or hunger, rapid heartbeat, sweating, lightheadedness and headache. Worsening symptoms can also include slurred speech, blurred vision and seizures.
Ileus
Ileus was officially listed on the Ozempic warning label in September 2023. This condition happens when the normal movement of food and waste through the intestines slows down or completely stops. But it happens without a physical blockage being present. Ileus may seem similar to a blockage in the intestines. But a true blockage occurs when something physically obstructs that passage. Common symptoms of ileus might include abdominal swelling, constipation, bloating, nausea and vomiting.
Pancreatitis
Pancreatitis occurs when enzymes or digestive juices attack the pancreas, causing inflammation. Pancreatitis symptoms often include fever, nausea, severe abdominal pain that worsens after eating, lowered blood pressure, jaundice and fluid buildup.
Pulmonary aspiration
Pulmonary aspiration is when a person accidentally breathes something (usually fluid or food) into their windpipe (trachea) and lungs. The foreign object causes coughing, trouble breathing and sometimes choking.
Ozempic delays gastric emptying, which can lead to regurgitation when someone is under sedation or anesthesia. As a result, the risk of pulmonary aspiration while sedated or under general anesthesia has been added to the warning label. The addition happened after the FDA had approved the drug and received negative reports during postmarketing experience.
Thyroid tumors
Medullary thyroid cancer (MTC) forms inside the medulla or thyroid gland and can spread to the lymph nodes and other organs. Symptoms of MTC often include enlarged glands, difficulty breathing or swallowing and changes in thyroid activity.
Adverse reactions to Ozempic
The most common adverse reactions reported on Ozempic’s warning label include:
- Abdominal pain
- Constipation
- Diarrhea
- Nausea and vomiting
If you are currently taking Ozempic or any other semaglutide drug and are experiencing new or worsening semaglutide side effects, consult your doctor as soon as possible. Medical treatment could reduce the severity of the side effects, but some Ozempic users find their symptoms continue even after they stop taking the drug.
What are potential unlisted side effects of Ozempic?
Ozempic manufacturers disclosed some side effects on the medication guide and prescribing information. However, some reports of severe gastrointestinal events and other health problems were not included on the warning label.
People are filing Ozempic lawsuits that claim Novo Nordisk knew or should have known about the risk of severe side effects like vision loss, gastroparesis, gastroenteritis, intestinal blockage, blood clots and other severe health problems but failed to warn users.
Gastroenteritis
Gastroenteritis occurs when the intestines and stomach become inflamed. Gastroenteritis, when viral, is also commonly referred to as the stomach flu. However, gastroenteritis may also be caused by certain types of medication. Some of the most common symptoms associated with gastroenteritis allegedly caused by Ozempic include:
- Dehydration
- Diarrhea
- Fever
- Headaches
- Muscle aches
- Nausea
- Stomach cramps
- Vomiting
Gastroenteritis can be far more severe than mild nausea and vomiting. The side effects of dehydration may require hospitalization, and continued symptoms can make it difficult to complete daily tasks.
Ileus
Since ileus wasn’t officially listed on the Ozempic warning label until September 2023, people who experienced this condition before September 2023 may have grounds for a legal case. In order for someone to be eligible, they’ll need to have proof of their ileus symptoms and how they were linked to taking Ozempic. However, state statutes of limitations may soon prohibit people in certain states from seeking compensation for this condition. Speak to an Ozempic lawyer soon to find out if you are eligible.
Intestinal blockages
This condition is when a blockage occurs in the intestines, making it nearly impossible for food and waste to move through the body as it needs to. Symptoms of intestinal blockages may include stomach cramping, vomiting, constipation and bloating.
While this may seem similar to ileus, an intestinal blockage happens when a physical blockage is present. Ileus happens when food and waste slow down or stop in spite of no physical blockage in the intestines.
Muscle loss
Taking Ozempic could lead to extreme muscle loss. This loss can occur when people lose weight quickly without maintaining exercise habits and a balanced diet. Also known as sarcopenia, severe muscle loss can lead to death. Some common symptoms of muscle loss could include:
- Decrease in muscle mass
- Difficulty performing daily activities
- Falls
- Loss of stamina
- Poor balance
- Trouble climbing stairs
- Walking slowly
Sarcopenia can also lead to other severe side effects and conditions, including insulin resistance, reduced bone density and lowered metabolism.
Stomach paralysis (gastroparesis)
Also known as stomach paralysis, gastroparesis slows down the movement of stomach muscles, preventing the stomach from emptying properly. This can lead to many debilitating symptoms and alleged Ozempic side effects, including:
- Abdominal bloating
- Abdominal pain
- Acid reflux
- Decreased quality of life
- Lack of appetite
- Nausea
- Severe dehydration
- Vomiting
Gastroparesis can be a very serious medical condition for which there is no cure. Motley Rice is currently representing people who developed severe gastroparesis after taking semaglutide medication. This ultimately led to painful cramping, nausea and vomiting that required hospitalization.
Learn more about how to file an Ozempic lawsuit for gastroparesis.
Vision changes and loss
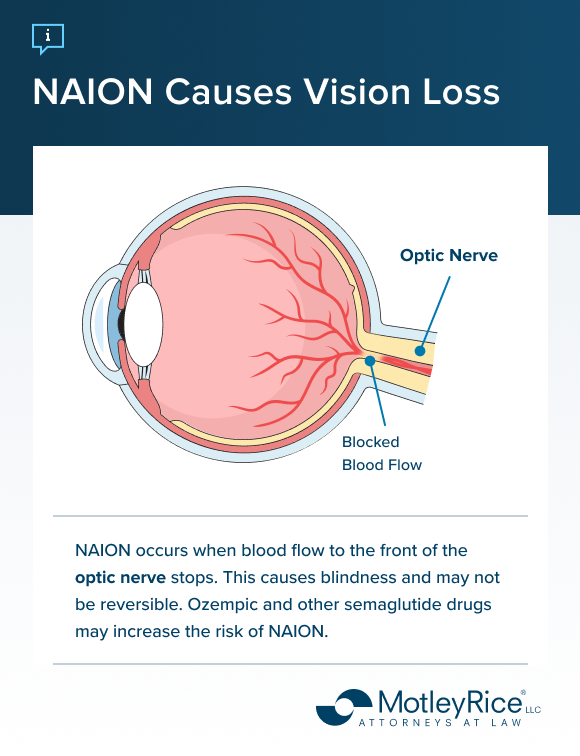
NAION (nonarteritic anterior ischemic optic neuropathy) is a condition where the arteries in the optic nerve head become inflamed. The inflammation may impact a person’s ability to see, potentially leading to blindness.
A 2024 study of more than 16,000 patients found that using semaglutide drugs like Wegovy or Ozempic was associated with increased diagnoses of NAION. The study looked at individuals taking semaglutide for weight loss or diabetes management. The increased risk was consistent among all users.
Ozempic has a warning on its label for diabetic retinopathy, a type of vision loss associated with diabetes. Because NAION and diabetic retinopathy may have similar symptoms, misdiagnoses are possible. If you or a loved one have experienced vision changes after taking Ozempic or other semaglutide drugs, consult with your doctor about the possibility of NAION.
Other potential unlisted Ozempic side effects
Consumers have reported other side effects after starting Ozempic or Wegovy, including:
- Malnutrition: You can become malnourished when your body is deprived of nutrients, minerals and vitamins. Symptoms of malnutrition include skin pigmentation changes, soft bones, bleeding gums, pale skin, rashes, thinning hair, achy joints and easy bruising.
- Blood clots and deep vein thrombosis: Blood clots are coagulated clumps of blood. A 2021 study reported that Ozempic use increased the risk of deep vein thrombosis (DVT), a condition caused by blood clotting deep in the body. DVT can be a very serious condition and can lead to pulmonary embolism.
Anyone taking Ozempic, Rybelsus®, Mounjaro™ or any semaglutide drug should not hesitate to contact their healthcare provider if they experience new or worsening side effects. It may also be in your best interests to connect with an Ozempic lawyer to explore your legal options.
Unexpected Ozempic side effects lead to lawsuits
The side effects not included on the Ozempic warning label are alarming. Thousands of people have allegedly been harmed, and records indicate manufacturer Novo Nordisk knew about these risks. Ozempic’s predecessor, Saxenda, caused similar side effects and was approved in 2014.
Tens of thousands of reports of health problems after taking Ozempic have been filed with the FDA Adverse Event Reporting System. Some noteworthy lawsuits include the following people affected by Ozempic:
- A 52-year-old woman taking weekly semaglutide injections experienced gastroparesis symptoms within one month of starting Ozempic.
- A 57-year-old woman taking dulaglutide injections every week for over a year began experiencing nausea, vomiting and bloating associated with delayed gastric emptying within three months of starting the prescription medication. These side effects improved when the patient stopped taking the dulaglutide.
- A 39-year-old woman needed to be hospitalized with significant gastrointestinal issues that have continued to affect her life even after she stopped taking Ozempic.
Patients suffering extreme side effects associated with Ozempic use have said the symptoms made it difficult or impossible to complete daily tasks or live life as they did before beginning Ozempic.
Legal action against Novo Nordisk may achieve justice and compensation for affected people. Compensation can help people cover financial losses, medical expenses and other relevant economic and non-economic damages.
FAQs about Ozempic side effects
What are the side effects of Ozempic for weight loss?
Ozempic labels include several possible side effects for those using the drug to lose weight or manage type 2 diabetes. Some of the most common side effects of Ozempic include abdominal pain, constipation, diarrhea, nausea, vision problems and vomiting. However, Ozempic users experiencing severe side effects may find that these symptoms last significantly longer or become a regular part of their daily lives. Severe side effects may also be life-threatening.
What are the long-term effects of Ozempic?
People could experience several long-term effects while taking Ozempic. Effects may include pancreatitis, gastrointestinal issues, thyroid cancer and vision changes. Consult with your doctor if you are experiencing severe side effects after taking Ozempic long-term. This might help you determine how long you should stay on Ozempic.
Do Ozempic side effects go away?
If you are experiencing side effects related to Ozempic, they may initially be mild. These mild side effects may resolve in several days or weeks but may continue with ongoing use of Ozempic or Wegovy. If you experience listed or unlisted side effects that significantly impact your life, talk with your doctor and seek medical attention.
Who can't take Ozempic?
According to the warning label, people who should not take Ozempic include:
- People with a history of pancreatitis
- People with type 1 diabetes (Ozempic is usually used to treat type 2 diabetes)
- People with a family history of medullary thyroid carcinoma (MTC)
- People with multiple endocrine neoplasia (MEN)
- People with a hypersensitivity reaction to semaglutide or the other inactive substances in Ozempic
- Pregnant women
- People under the age of 18 (Ozempic’s safety and effectiveness in children under 18 is unknown)
Our medical drug litigation experience
Our law firm has years of experience representing the interests of patients who became seriously ill and endured debilitating side effects associated with prescribed medications. We also represent Wegovy and Ozempic patients. If you or someone you love experienced severe symptoms related to the use of these diabetes and weight loss drugs, our personal injury attorneys may be able to help. We have the knowledge and resources to help you demand justice. Our team can help you:
- Investigate whether Ozempic could be the cause of your vision changes, gastroparesis, intestinal blockages or other side effects
- Review whether Novo Nordisk failed to warn you about the potential off-label side effects adequately
- Help build and file your case if you qualify
Read more on our medical drug experience.
Do not stop taking a prescribed medication without first consulting with your doctor. Discontinuing a prescribed medication without your doctor's advice can result in injury or death. Ozempic, Wegovy, Mounjaro, Saxenda and Rybelsus remain approved by the U.S. Food and Drug Administration.
Key takeaways
What are the listed side effects of Ozempic?
What are potential unlisted side effects of Ozempic?
Unexpected Ozempic side effects lead to lawsuits
FAQs about Ozempic side effects
Our medical drug litigation experience
- Sources
- American Cancer Society. Signs and Symptoms of Pancreatic Cancer.
- American Thyroid Association. Medullary Thyroid Cancer.
- Cleveland Clinic. Bowel Obstruction.
- Cleveland Clinic. Gallbladder Disease.
- Cleveland Clinic. Medullary Thyroid Cancer (MTC).
- Cleveland Clinic. Sarcopenia.
- Drugs.com. Can Ozempic cause stomach paralysis or bowel injury?
- The George Washington University Hospital. Biliary Disease.
- Hathaway JT, Shah MP, Hathaway DB, Zekavat SM, Krasniqi D, Gittinger JW, et al. Risk of nonarteritic anterior ischemic optic neuropathy in patients prescribed semaglutide. JAMA Ophthalmol [Internet]. 2024 Jul 3.
- Healthline. Ozempic Can Cause Major Loss of Muscle Mass and Reduce Bone Density.
- Johns Hopkins Medicine. Malnutrition.
- Johns Hopkins Medicine. Pancreatitis.
- Kalas MA, Galura GM, McCallum RW, Medication-Induced Gastroparesis: A Case Report, J Investig Med High Impact Case Rep. 2021 Jan-Dec; 9.
- Mayo Clinic. Diabetic Retinopathy.
- Mayo Clinic. Gastroparesis.
- Mayo Clinic. Hypoglycemia.
- Mayo Clinic. Intestinal Obstruction.
- Mayo Clinic. Viral gastroenteritis (stomach flu).
- Medical News Today. What to know about the different types of hypersensitivity reactions.
- MedPage Today. FDA Adds New Warning to GLP-1 Drugs.
- Merck Manual. Drug-Related Gastroenteritis and Chemical-Related Gastroenteritis.
- National Eye Institute. At a Glance: Diabetic Retinopathy.
- National Kidney Foundation. Acute Kidney Injury (AKI).
- National Library of Medicine. Ileus.
- National Library of Medicine. Non-Arteritic Anterior Ischemic Optic Neuropathy.
- Nuvance Health. Liver, Pancreas and Gallbladder Disorders (Hepatobiliary Disease).
- Ozempic. Frequently Asked Questions.
- U.S. Food and Drug Administration. FDA Adverse Event Reporting System (FAERS) Public Dashboard.
- U.S. Food and Drug Administration. HIGHLIGHTS OF PRESCRIBING INFORMATION: OZEMPIC (semaglutide) injection, for subcutaneous use.
- U.S. Food and Drug Administration. Ozempic Highlights of Prescribing Information.
- U.S. Food and Drug Administration. OZEMPIC (semaglutide) injection, for subcutaneous use Initial U.S. Approval: 2017.
- U.S. Food and Drug Administration. Saxenda Highlights of Prescribing Information.
- University of Pennsylvania Health System. Blood Clots Symptoms and Causes.
- Yin DG, Ding LL, Zhou HR, Qiu M, Duan XY. Comprehensive analysis of the safety of semaglutide in type 2 diabetes: a meta-analysis of the SUSTAIN and PIONEER trials. Endocr J. 2021;68(6):739–42.
Start Your Motley Rice Consultation in Simple Steps
Submit Information
Call us or fill out our online form with the details of your potential case.
Case Review
Our team reviews your information to assess your potential case.
Case Consultation
Talk with us about next steps.


